Synthetic SPECIES Developed for Use as a Confinable Gene Drive
Researchers create novel CRISPR-based fly species as a new method of controlling gene drive spread
June 7, 2021
By Mario Aguilera
CRISPR-based technologies offer enormous potential to benefit human health and safety, from disease eradication to fortified food supplies. As one example, CRISPR-based gene drives, which are engineered to spread specific traits through targeted populations, are being developed to stop the transmission of devastating diseases such as malaria and dengue fever.
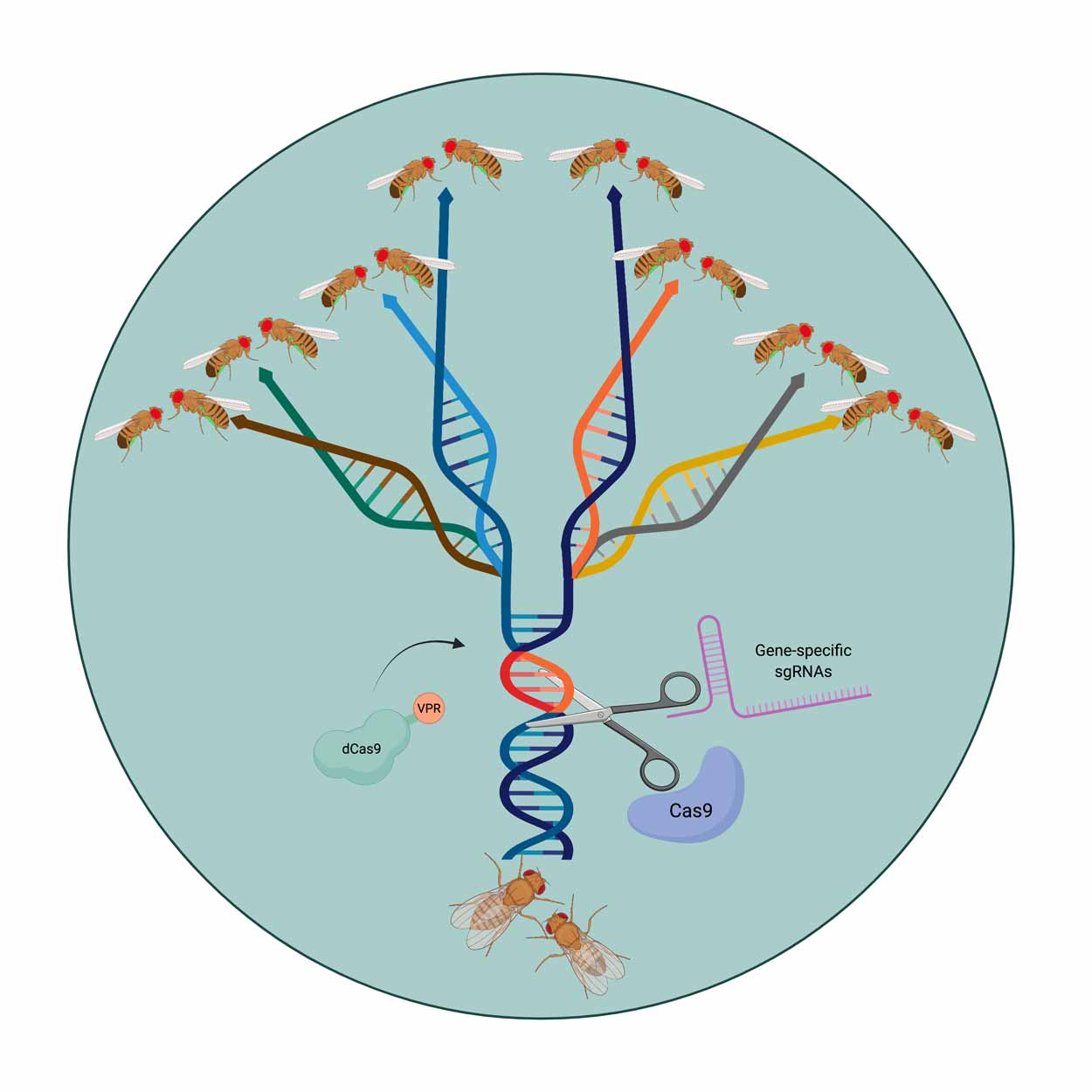
UC San Diego scientists have modified the genome of fruit flies using CRISPR-based technologies to create eight reproductively isolated species. In the future, this technique can be adapted to other organisms including plants, insects and vertebrates to provide new biocontrol opportunities.
But many scientists and ethicists have raised concerns over the unchecked spread of gene drives. Once deployed in the wild, how can scientists prevent gene drives from uncontrollably spreading across populations like wildfire?
Now, scientists at the University of California San Diego and their colleagues have developed a gene drive with a built-in genetic barrier that is designed to keep the drive under control. Led by molecular geneticist Omar Akbari's lab, the researchers engineered synthetic fly species that, upon release in sufficient numbers, act as gene drives that can spread locally and be reversed if desired.
The scientists describe their SPECIES (Synthetic Postzygotic barriers Exploiting CRISPR-based Incompatibilities for Engineering Species) development as a proof-of-concept innovation that could be portable to other species such as insect disease vectors. Spreading gene drives that limit pests that feast on valuable food crops is another example of a potential SPECIES application.
"Gene drives can potentially spread beyond intended borders and be hard to control. SPECIES offers a way to control populations in a very safe and reversible manner," said Akbari, a UC San Diego Division of Biological Sciences associate professor and senior author of the paper, which is published in the journal Nature Communications.
The idea behind the creation of SPECIES is reflective of the formation of new species in nature. As members of a single species separate over time, due to, for example, a new land formation, earthquake separation or other geological event, a new species eventually can evolve from the physical disconnection. If the new species eventually returns to mate with the original species, they could produce unviable offspring due to biological changes following the separation through a natural phenomenon known as reproductive isolation.
Working in the fly species Drosophila melanogaster, UC San Diego researchers and their colleagues at the California Institute of Technology, UC Berkeley and the Innovative Genomics Institute used CRISPR genetic-editing technologies to develop flies encoding SPECIES systems that are reproductively incompatible with wild versions of D. melanogaster.
"Even though speciation happens consistently in nature, creating a new artificial species is actually a pretty big bioengineering challenge," said Anna Buchman, the lead author of the paper. "The beauty of the SPECIES approach is that it simplifies the process, giving us a defined set of tools we need in any organism to elegantly bring about speciation."
Conceptually, when SPECIES are deployed in the wild in sufficient numbers, they can controllably drive through a population and replace all of their wild counterparts as they spread. Using malaria as an example, SPECIES mosquitoes could be developed with a genetic element that makes them incapable of transmitting malaria.
"You can spread an anti-malaria SPECIES into a target population in a confinable and controllable way," said Akbari. "Since SPECIES are incompatible with wild-type mosquitoes, their populations can be controlled and reversed by limiting their threshold population below 50 percent. This gives you the ability to confine and reverse its spread if desired."
As the SPECIES barrier completes its role in temporarily replacing wildtype populations, their numbers can be reduced with the reintroduction of wild type populations.
"This essentially allows us to harness all of the power of gene drives—like disease elimination or crop protection—without the high risk of uncontrollable spread," said Akbari.
Coauthors of the paper include Anna Buchman, Isaiah Shriner (former UC San Diego undergraduate student), Ting Yang, Junru Liu (current Biological Sciences PhD student), Igor Antoshechkin, John Marshall, Michael Perry and Omar Akbari.
The research was funded in part by UC San Diego lab startup funds and a DARPA Safe Genes Program Grant (HR0011-17-2-0047).
Note: Akbari is a co-founder of Agragene Inc. with equity.
