UC San Diego Biologists Gain New Insights into Brain Circuit Wiring
February 14, 2011
By Kim McDonald
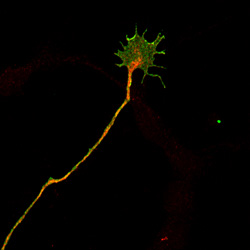
UC San Diego biologists discovered how growth cones at the tips of growing nerves are guided to wire the developing brain.
Credit: Yimin Zou, UCSD
Neurobiologists at UC San Diego have discovered new ways by which nerves are guided to grow in highly directed ways to wire the brain during embryonic development.
Their finding, detailed in a paper in the February 15 issue of the journal Developmental Cell, provides a critical piece of understanding to the longstanding puzzle of how the human brain wires itself into the complex networks that underlie our behavior.
The discovery concerns the movements of a highly sensitive and motile structure at the tips of growing nerves called a growth cone. For more than a century, biologists have known that growth cones find their targets by detecting chemical cues in the developing nervous system. They do that by responding to gradients of chemical concentration and steering nerve cells either up or down the gradient to eventually find the right targets to make the proper nerve connections that then establish neuronal networks.
While many of these chemical guidance cues have been identified over the past decade, scientists still don’t fully understand how the growth cone picks up small concentration differences in the developing embryo or how guidance cues enter the growth cone to regulate the cellular machinery to turn growth cones in one direction or another.
Yimin Zou, an associate professor of neurobiology at UC San Diego, and his colleagues had previously shown that a family of proteins known as “Wnt morphogens” provide the directional cues for the wiring of circuits in many parts of the developing brain.
“These morphogens are often strategically placed in important organizing centers of the developing nervous system and play a major role in sculpting brain connections,” said Zou.
In their latest paper, Zou and his UCSD colleagues, Beth Shafer, Keisuke Onishi, Charles Lo and Gulsen Colakoglu, report their discovery that Wnt proteins steer the growth cone by stimulating planar cell polarity signaling.
“Planar cell polarity or PCP refers to the polarized structures and functions of a sheet of epithelial cells along the plane of the tissue,” he said. “The direction of our skin hair in our backs, for example, is polarized to point down from our head and a highly conserved genetic program, the PCP signaling system, ensures this type of tissue organization in our skin as well as many other parts of our body.”
The UCSD research team found that the growth cone is equipped with all the PCP components necessary to steer extensions of nerve cells, or axons, to their proper targets within the Wnt gradients.
“This study reveals a novel type of environmental signal which the growth cone responds to, previously unknown to developmental neurobiologists, the tissue polarity signals,” said Zou. “Tissue polarity is a long lasting structural feature and may provide organizational information to axonal connections while the brain is being wired up. Because PCP signaling is essential for the beautifully organized structures in the brain, the brain may owe a large part of its stunning axonal organization to the function of PCP signaling. PCP signaling relies heavily on cell-cell interactions, which may pave way for developmental neuroscientists to understand how groups of neurons organize their axons into exquisite patterns.”
The UCSD researchers also found that one of the PCP components, Vangl2, is highly enriched on the tips of growing filopodia on the growth cone.
“The filopodia are the motile structures that explore the environment,” said Zou. “It has been long speculated that the long filopodia can extend the span of the growth cone to sample larger concentration drops. The localization of Vangl2 on growth cone tips suggests that the tips are more sensitive to guidance cues than the rest of the growth cone.”
“This paper not only reveals the profound logic of brain wiring mechanisms but also provides satisfactory and in-depth mechanistic insights,” he added. “With these new insights and tools at hand, one can now move on to design experiments to ask the next level questions, such as the cell biological mechanisms of growth cone steering. These findings will also provide new methods for nervous system repair and regeneration.”
The study was mainly funded by a grant from the National Institutes of Health.
