Calcium Signals Found to Guide Nerve Cell Development
MARCH 8, 2001
By: Kim McDonald
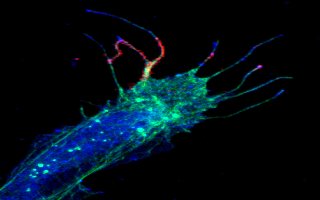
Color image showing red and fuchsia calcium bursts in filopodia from a frog spinal nerve cell. Credit: Timothy Gomez, UCSD
Biologists at the University of California, San Diego have discovered that growing nerve cells in the developing embryo are guided to their proper targets by bursts of intracellular calcium that probe what's ahead and send back information to the cells in a kind of biological Morse code.
In a paper published in the March 9 issue of Science, the researchers report their discovery that finger-like projections on growing nerve cells, known as filopodia, sample the environment and generate tiny bursts of calcium at their tips that send back information in a manner similar to an FM radio, enabling the neurons to wire up proper connections in the developing brain, spinal cord, and other parts of an animal's body.
"These bursts, which usually occur at the very ends of the filopodia, are extremely brief-about a 300-millisecond pulse-which may explain why they were undetected in earlier studies," says Timothy M. Gomez, an assistant professor of anatomy at University of Wisconsin Medical School who headed the research while a postdoctoral fellow at UCSD. "Since many other types of cells have these finger-like projections, we believe that these brief calcium bursts may be a universal signaling mechanism for motile cells."
"Many kinds of birth defects and a large fraction of spinal cord defects appear to be associated with problems in the formation of nerve connections in the developing embryo," says Nicholas C. Spitzer, a professor of biology at UCSD, commenting on the wider implications of the discovery. "We want to understand how the normal brain is put together, so we can understand the cases in which it is not put together correctly."
In addition to Gomez and Spitzer, who headed the laboratory in which the research was conducted, others involved in the discovery were Mu-ming Poo, a UCSD professor of biology now at the University of California, Berkeley; and Estuardo Robles, an undergraduate at UCSD who is now a graduate student at the University of Wisconsin at Madison. The team's work was supported by the National Institute of Neurological Disorders and Stroke.
Neurobiologists have long known that certain molecules influence the direction of the growth of neurons. But until now, scientists had few clues about how filopodia send their signals to the motile tips of neurons, called growth cones, or how they controlled the growing nerve cells' movements.
When the finger-like projections of filopodia sprout from their growth cones, they sweep the area ahead of them searching for molecules and other cues released by target cells. As the filopodia steer the growth cones to their target cells, long axons are formed, which transfer chemical signals between cells. In the mature nervous system, the growth cones are transformed into synapses, the communication connections between the nerve cells.
"The movement, the steering, the guiding in the growing nerve cell is all done by the growth cones," says Spitzer. "The growth cones are the pioneers, leading the way for the growth of the axons behind them."
To conduct their study, the UCSD researchers used spinal nerve cells from developing frog embryos, which can be generated in large quantities in the laboratory and grow rapidly. Introducing a fluorescent calcium marker into the cells, which were kept alive in culture dishes, the scientists were able to record with a camera attached to a microscope the rapid calcium bursts at the tips of the filopodia as they traveled down to the growth cone within a second.
The researchers were also able to determine how the calcium bursts affected the movements of the growth cones. By exposing the cells to eight different chemical environments in the culture dish, some more favorable to nerve cell growth than others, they discovered that the frequency of the bursts varied with the kinds and concentrations of chemicals in the culture dishes. They also found that the calcium burst frequencies varied with the positioning of the growth cones and filopodia, and that experimentally produced calcium bursts on one side of the growth cone caused the reorientation of nerve cell growth. These frequency modulated, or FM burst signals, send information to the growth cones about the environment ahead in a manner analogous to the information transmitted on FM radio.
"When they sense a different environment, the calcium bursts occur at a different frequency and this, in turn, influences growth cone turning toward or away from particular molecular cues that shape their connections," says Spitzer. "Just like our fingers on the FM dial on the radio, these cues change the FM dial on the growth cone to produce these highly relevant signals."
