Discovery Sheds Light on Mechanisms that Allow Cells to Steer Directly Toward Chemical Cues
May 30, 2002
By: Kim McDonald
Biologists at the University of California, San Diego have found that two genes associated with the development of human cancer play a central role in orienting a cell’s “internal compass,” guiding white blood cells with missile-like precision to the sites of infections and leading other types of cells directly to nutrients and a myriad of other important chemical cues.
Dictyostelium cells move toward chemical attractants“Many cells within our body are able to sense chemical signals over long distances and move to the source of these signals,” says Richard A. Firtel, a professor of biology and director of the Center for Molecular Genetics at UCSD. “For example, when a wound becomes infected, white blood cells rush to the site of the wound in the body’s first major response to fighting the infection. The white blood cells are able to sense the bacteria because the bacteria release small molecules that are recognized by proteins on the surface of the white blood cell. Similarly, breast cancer cells can migrate out of the tumor in the direction of blood vessels, leading to metastasis and secondary sites of tumor formation.”
Scientists have learned much about the chemical cues that elicit these responses in cells. But until now, the mechanisms cells use to steer themselves to these attractants had been poorly understood.
Firtel and his colleagues—UCSD postdoctoral researchers Satoru Funamoto and Reudi Meili; Susan Lee, a research associate; and Lisa Parry, an undergraduate student—elucidated how this internal directional compass in cells works by studying Dictyostelium discoideum, a simple social amoeba and model genetic system that exhibits many of the properties of white blood cells. The scientists first determined that chemotaxis in Dictyostelium is controlled by two genes responsible for the production of a pair of proteins—phosphatidylinositol-3 kinase, or PI3K, and phosphatidylinositol-3 phosphatase, or PTEN, the latter of which is associated with half of all human tumors. Mutations in the two genes that control the production of these proteins result in cells that have little chemotaxis ability.
|
“Instead of moving toward the source of the signal, these cells wander about, having lost their internal sense of direction,” says Firtel, who is also chair of the Section of Cell and Developmental Biology of UCSD’s Division of Biological Sciences.
Using a fluorescent tag attached to the PI3K molecules to visualize the movement of this protein in living cells, the scientists then determined how the steering mechanism worked. They found that normal cells steered toward chemical attractants by first accumulating PI3K within the part of the cell closest to the direction of the chemical gradient. Once localized there, the protein acts as both an internal compass and steering mechanism.
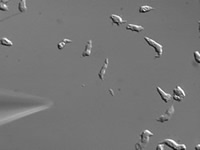 |
| Chemical begins attracting Dictyostelium cells |
“At rest, PI3K is uniform in the cell,” explains Firtel. “So PI3K functions as a compass by going to the side of the cell closest to the direction it wants to move. That side of the cell is the direction in which the level of the chemical attractant is the highest. This is how a cell senses direction.”
The researchers also discovered that when cells lacked PI3K, they were unable to effectively organize the cellular motors to make the cell move. In addition, when PI3K is attached to uniformly to the entire inside of the cell’s membrane, the scientists found that the cells no longer put out a new front only in the direction of the chemical attractants.
“The result is that instead of a single front, the cell acts as if it has many fronts moving in many directions at the same time,” says Firtel. “It’s as if the cell has many drivers, all wanting to lead the cell in a different direction.”
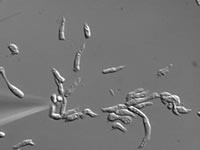 |
| Fifteen minutes later cells migrate to chemical source |
Perhaps the most unexpected aspect of the discovery is that the two proteins that control the cell’s directional ability, as well as the genes responsible for producing them, also control the development of human cancer.
“PTEN is a regulator of PI3K and, loss of it from cells within our body leads to human cancer,” explains Firtel. “PTEN basically works in normal cells to put the brakes on PI3K. When PI3K becomes overactive, you have unregulated cell growth and the cell becomes cancerous.”
The researchers demonstrated in their experiments that PTEN controls cell movement in a manner similar to the way it controls the normal growth of cells by inhibiting the activity of PI3K. During chemotaxis, PTEN inhibits the PI3K function in specific sites within the cell, keeping the cell “focused” on going in the right direction. The scientists also discovered that when PI3K is concentrated at the front end of a chemotaxing cell, PTEN is concentrated on the sides and back part of the cell. In this way, the compass, or PI3K, can only work at the front, in the direction the cell wants to move.
“This suggests that PTEN works much like the blinders on a racehorse, restricting its field of vision, so that the cells move efficiently in the direction the compass is pointing,” says Firtel. “Cells in which PTEN activity is decreased lose their sense of direction because they have lost these blinders and they make incorrect turns.”
Firtel notes that the primary function of these two proteins evolutionarily is to provide the steering mechanisms for normal cellular functions, but that many cancer cells have co-opted this mechanism to metastasize or spread cancerous cells throughout the body.
“There is growing evidence that many cancer cells metastasize using the same process of chemotaxis,” he adds. “So, understanding how a white blood cell or Dictyostelium cell moves will provide insights into how cancer cells metastasize. Cancer cells also need to move and they need to move directionally. Chemicals called chemokines within the blood or tissues outside the tumor are what attract the metastasizing cancer cells.”
Information about the steering mechanisms that control the accumulation of white blood cells within the body could also provide medical researchers with new tools to control the pain and swelling associated with arthritis and injuries that take longer to heal because of excessive inflammation.
“When we have a site of inflammation or an arthritic attack, white blood cells rush to these sites just as they would to the site of an infection,” explains Firtel. “But in this instance, instead of fighting a bacterial infection, the white blood cells trigger other responses, resulting in swelling and pain.”
Financial support for the studies that led to these discoveries was provided by grants from the U.S. Public Health Service.
