Study Suggests Disruptive Effects of Anesthesia on Brain Cell Connections Are Temporary
July 28, 2014
By Kim McDonald
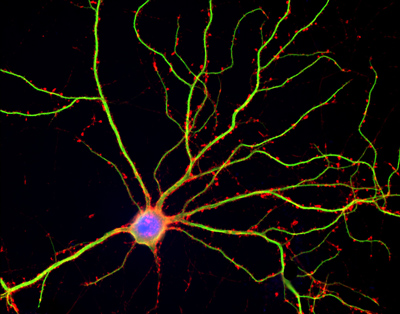
Hippocampal neuron in culture. Dendrites are green, dendritic spines red, and DNA in cell’s nucleus is blue.
Shelley Halpain, UC San Diego
A study of juvenile rat brain cells suggests that the effects of a commonly used anesthetic drug on the connections between brain cells are temporary.
The study, published in this week’s issue of the journal PLOS ONE, was conducted by biologists at the University of California, San Diego and Weill Cornell Medical College in New York in response to concerns, arising from multiple studies on humans over the past decade, that exposing children to general anesthetics may increase their susceptibility to long-term cognitive and behavioral deficits, such as learning disabilities.
An estimated six million children, including 1.5 million infants, undergo surgery in the United States requiring general anesthesia each year and a least two large-scale clinical studies are now underway to determine the potential risks to children and adults.
“Since these procedures are unavoidable in most cases, it’s important to understand the mechanisms associated with the potentially toxic effects of anesthetics on the developing brain, and on the adult brain as well,” said Shelley Halpain, a professor of biology at UC San Diego and the Sanford Consortium for Regenerative Medicine, who co-headed the investigation. “Because the clinical studies haven’t been completed, preclinical studies, such as ours, are needed to define the effects of various anesthetics on brain structure and function.”
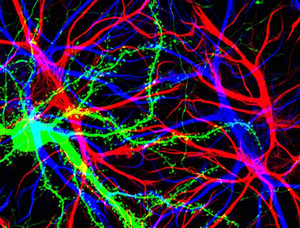
Hippocampal cells with neuron in green showing hundreds of the small protrusions known as dendritic spines. The dendrites of other dendrites are labeled in blue, and adjacent glial cells are shown in red.
Barbara Calabrese, UC San Diego
“There is concern now about cognitive dysfunction from surgery and anesthesia–how much these effects are either permanent or slowly reversible is very controversial,” said Hugh Hemmings, Jr., chair of anesthesiology at Weill Cornell and the study’s other senior author. “It has been suggested recently that some of the effects of anesthesia may be more lasting than previously thought. It is not clear whether the residual effects after an operation are due to the surgery itself, or the hospitalization and attendant trauma, medications and stress–or a combination of these issues.”
However, he added, “There is evidence that some of the delayed or persistent cognitive effects after surgery are not primarily due to anesthesia itself, but more importantly to brain inflammation resulting from the surgery. But this is not yet clear.”
The team of biologists examined one of the most commonly used general anesthetics, a derivative of ether called “isoflurane” used to maintain anesthesia during surgery.
“Previous studies in cultured neurons and in the intact brains of rodents provided evidence suggesting that exposure to anesthetics might render neurons more susceptible to cell death through a process called ‘apoptosis’,” said Halpain. “While overt cell death could certainly be one way to explain any long-lasting neurocognitive consequences of general anesthesia, we hypothesized that there could be other cellular mechanisms that disrupt neural circuits without inducing cell death per se.”
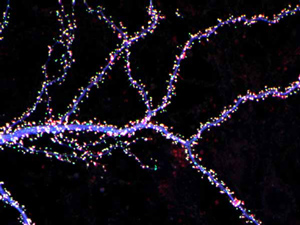
Hippocampal neuron from rodent brain with dendrites shown in blue. The hundreds of tiny magenta, green and white dots are the dendritic spines of excitatory synapses.
Barbara Calabrese, UC San Diego
One such mechanism, she added, is known as “synaptotoxicity.” In this mechanism of neural-circuit disruption, the “synapses,” or junctions between neurons, become weakened or shrink away due to some factor that injures the neurons locally along their axons (the long processes of neurons that transmit signals) and dendrites (the threadlike extensions of neurons that receive nerve signals) without inducing the neurons themselves to die.
In the experiments at UC San Diego headed by Jimcy Platholi, a postdoctoral researcher in Halpain’s lab who is now at Weill Cornell, the scientists used neurons from embryonic rats taken from the hippocampus, a part of the mammalian forebrain essential for encoding newly acquired memories and ensuring that short-term memories are converted into long-term memories. The researchers cultured these brain cells in a laboratory dish for three weeks, allowing the neurons time to mature and to develop a dense network of synaptic connections and “dendritic spines”–specialized structures that protrude from the dendrites and are essential mediators of activity throughout neural networks.
“Evidence from animal studies indicates that new dendritic spines emerge and existing spines expand in size during learning and memory,” explained Halpain. “Therefore, the overall numbers and size of dendritic spines can profoundly impact the strength of neural networks. Since neural network activity underlies all brain function, changes in dendritic spine number and shape can influence cognition and behavior.”
Using neurons in culture, rather than intact animal brains, allowed the biologists to take images of the synapses at high spatial resolution using techniques called fluorescence light microscopy and confocal imaging. They also used time-lapse microscopy to observe structural changes in individual dendritic spines during exposure to isoflurane. Karl Herold, a research associate in the Hemmings laboratory and a co-author of the study, performed some of the image analysis.
“Imaging of human brain synapses at this level of detail is impossible with today’s technology and it remains very challenging even in laboratory rodents,” said Halpain. “It was important that we performed our study using rodent neurons in a culture dish, so that we could really drill down into the subcellular and molecular details of how anesthetics work.”
The researchers wondered whether brief exposure to isoflurane would alter the numbers and size of dendritic spines, so they applied the anesthetic to the cultured rat cells at concentrations and durations (up to 60 minutes) that are frequently used during surgery.
“We observed detectable decreases in dendritic spine numbers and shape within as little as 10 minutes,” said Halpain. “However this spine loss and shrinkage was reversible after the anesthetic was washed out of the culture.”
“Our study was reassuring in the sense that the effects are not irreversible and this fits in with known clinical effects,” said Hemmings. “For the most part, we find that the effects are reversible.”
“We clearly see an effect–a very marked effect on the dendritic spines–from use of this drug that was reversible, suggesting that it is not a toxic effect, but something more relevant to the pharmacological actions of the drug,” he added. “Connecting what we found to the cognitive effects of isoflurane will require much more detailed analysis.”
The team plans to follow up its study with future experiments to probe the molecular mechanisms and long-lasting consequences of isoflurane’s effects on neuron synapses and examine other commonly-used anesthetics for surgery.
A copy of the paper can be accessed at http://dx.plos.org/10.1371/journal.pone.0102978 The study was supported by grants from the National Institutes of Health (MH087823 and GM58055).
