Glowing, Blinking Bacteria Reveal How Cells Synchronize Biological Clocks
September 8, 2011
By Kim McDonald
Green fluorescent protein causes the E. coli to glow when the cells' clock is activated.
UC San Diego
Biologists have long known that organisms from bacteria to humans use the 24 hour cycle of light and darkness to set their biological clocks. But exactly how these clocks are synchronized at the molecular level to perform the interactions within a population of cells that depend on the precise timing of circadian rhythms is less well understood.
To better understand that process, biologists and bioengineers at UC San Diego created a model biological system consisting of glowing, blinking E. coli bacteria. This simple circadian system, the researchers report in the September 2 issue of Science, allowed them to study in detail how a population of cells synchronizes their biological clocks and enabled the researchers for the first time to describe this process mathematically.
“The cells in our bodies are entrained, or synchronized, by light and would drift out of phase if not for sunlight,” said Jeff Hasty, a professor of biology and bioengineering at UC San Diego who headed the research team. “But understanding the phenomenon of entrainment has been difficult because it’s difficult to make measurements. The dynamics of the process involve many components and it’s tricky to precisely characterize how it works. Synthetic biology provides an excellent tool for reducing the complexity of such systems in order to quantitatively understand them from the ground up. It’s reductionism at its finest.”
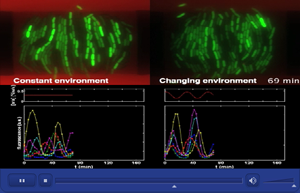
At right, periodic pulses of arabinose (in red) act like day and night cycles to simulate how the blinking bacteria synchronize their biological clocks. At left, a simulation of bacteria in constant darkness reveals how the blinking bacteria are unable to synchronize their biological clocks. The in-phase and out-of-phase oscillations of the biological clocks are shown by the bottom two graphs.
UC San Diego
To study the process of entrainment at the genetic level, Hasty and his team of researchers at UC San Diego’s Biocircuits Institute combined techniques from synthetic biology, microfluidic technology and computational modeling to build a microfluidic chip with a series of chambers containing populations of E. coli bacteria. Within each bacterium, the genetic machinery responsible for the biological clock oscillations was tied to green fluorescent protein, which caused the bacteria to periodically fluoresce.
To simulate day and night cycles, the researchers modified the bacteria to glow and blink whenever arabinose–a chemical that triggered the oscillatory clock mechanisms of the bacteria–was flushed through the microfluidic chip. In this way, the scientists were able to simulate periodic day-night cycles over a period of only minutes instead of days to better understand how a population of cells synchronizes its biological clocks.
Hasty said a similar microfluidic system in principal could be constructed with mammalian cells to study how human cells synchronize with light and darkness. Such genetic model systems would have important future applications since scientists have discovered that problems with the biological clock can result in many common medical problems from diabetes to sleep disorders.
Other members of Hasty’s team included Lev Tsimring, associate director of the BioCircuits Institute, and bioengineering graduate students Octavio Mondragon, Tal Danino and Jangir Selimkhanov. Their research was supported by grants from the National Institute of General Medical Sciences and the San Diego Center for Systems Biology.
