Discovery May Shed Light on Why Some HIV-Positive Patients Have More Virus
September 24, 2012
By Kim McDonald
Biologists at UC San Diego have unraveled the anti-viral mechanism of a human gene that may explain why some people infected with HIV have much higher amounts of virus in their bloodstreams than others.
Their findings, detailed in a paper in this week’s advance online issue of the journal Nature, could also shed light on the mystery of why some people with HIV never develop symptoms of AIDS. The biologists found that a gene called Human Schlafen 11 produces a protein that inhibits the replication of HIV in infected human cells by blocking the ability of the host cell to synthesize viral proteins.
“Some people with HIV develop AIDS rapidly and others can be HIV positive for decades and never really develop any symptoms of the disease,” said Michael David, a professor of biology at UC San Diego, who headed the research team. “It’s still unclear why that is, but one possibility is that the genetic variations in this protein, like in many other viral restriction factors, account for the differences in the susceptibility to the virus.”
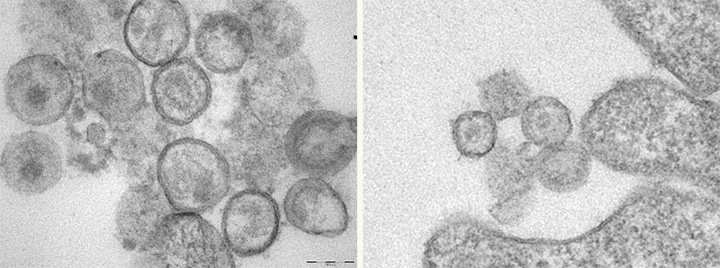
Cells without the Human Schlafen 11 gene have more HIV virus-like particles (seen as round dots at left) than cells at right that express the gene.
Manqing Li, UC San Diego
Cells without the Human Schlafen 11 gene have more HIV virus-like particles (seen as round dots at left) than cells at right that express the gene. Credit: Manqing Li, UC San Diego Because Human Schlafen 11 specifically blocks synthesis of HIV proteins, the researchers are conducting further studies to see if variations in the Human Schlafen 11 gene can be correlated with disease progression in HIV infected individuals. If that turns out to be the case, the discovery could one day lead to the development of a diagnostic test for HIV infected individuals that would inform them of their likelihood of developing AIDS or, better yet, the development of a therapeutic drug that would prevent HIV infected individuals from ever developing AIDS.
“If it’s possible for the human cell to inhibit the synthesis of viral programs without affecting the synthesis of cellular proteins, it’s possible that at some point a drug can do that, too,” said David. “But our discovery is just the tip of the iceberg. There’s a lot more work to be done. Whether this will have diagnostic or therapeutic value remains to be seen.”
Human Schlafen 11 is member of a family of six genes in humans and nine genes in mice that are induced in mammalian cells in response to various kinds of infection, specifically infections that result in the release of anti-viral proteins called interferons. The first Schlafen gene was discovered in mice at UC San Diego in 1998 by Steve Hedrick, a professor of biology.
David said his laboratory had spent the past eight years trying to figure out what role Human Schlafen 11 plays in human cells before discovering its unique role. He added that they were intrigued when Manqing Li, a project scientist in the lab, discovered that the Human Schlafen 11 protein was missing in a cell line used to produce large amounts of virus in the laboratory. “When we put Schlafen 11 back into the cell line, we got over 90 percent inhibition of virus output,” David said, confirming that the gene was critical to inhibiting virus replication.
David said that while Schlafen genes have been known for many years, his laboratory’s discovery is the first to shed light on how they work at the molecular level. His team is now collaborating with several groups to determine if other Human Schlafen genes have an anti-viral effect against other viruses, such as those that cause influenza and dengue fever.
The researchers are also collaborating with scientists who oversee tissue banks containing DNA samples from thousands of individuals infected with HIV to determine whether variations in the genetic sequences of the Human Schlafen 11 gene can be correlated with the development of clinical symptoms in those individuals. David’s team is part of a collaboration called HIV Immune Networks Team or HINT (http://hint.org/), which is funded by NIAID at the National Institutes of Health to “use systems biology approaches to reveal how the early immune response defends against HIV-1 infection with a view toward blocking virus.”
UC San Diego biologists Manqing Li, Elaine Kao, Xia Gao and Hilary Sandig in David’s laboratory worked on the experiments that led to the discovery. Other co-authors of the paper included Mariana Pavon-Eternod, Thomas Jones and Tao Pan of the University of Chicago; and Sebastien Landry and Matthew Weitzman of The Salk Institute for Biological Studies.
The research was supported by grants from the National Institutes of Health (AI81019, AI074967, P01AI090935, R01GM101982 and R21AI088490). Anyone interested in licensing this technology should contact the UC San Diego Technology Transfer office at [email protected]
