Emily Troemel
Research
How do pathogens attack their hosts, and how do hosts defend themselves against these attacks? We are using the roundworm C. elegans to study these questions of host/pathogen interactions in the intestine. C. elegans provides a powerful and tractable system for research. The worm has intestinal cells that are similar in structure to human intestinal cells, yet C. elegans is transparent and easy to study in lab. C. elegans lacks a professional immune system, and instead relies on 'non-professional' immune cells like epithelial cells to fight off pathogen attack. It is increasingly appreciated that epithelial cells in mammals are involved in detecting and responding to pathogens, and these cells can also be drivers of inflammatory disorders. It is critical to understand more about immunity in these cells, and what we find in C. elegans host/pathogen interactions in epithelial cells may help to understand and treat both infectious and inflammatory diseases in humans.
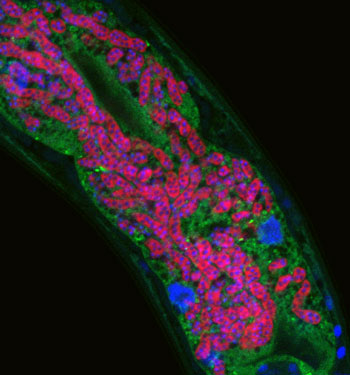
Microsporidia infecting C. elegans intestine – microsporidia are labeled in red, C. elegans intestine labeled in green, and DNA labeled in blue.
Our research focuses on two distinct pathogens, both of which are natural intracellular pathogens isolated from C. elegans in the wild. The first pathogen is a species of microsporidia that we named Nematocida parisii, or nematode-killer from Paris (intestinal infection of C. elegans intestine with N. parisii shown in figure image). Microsporidia are poorly understood fungal pathogens that infect most animals, and can cause problems for immunocompromised humans, as well as for agriculturally important animals like honeybees. The second pathogen we study is a natural viral pathogen of C. elegans from Orsay, France that also infects the intestine. Like coronaviruses, the Orsay virus is a positive-sense, single-stranded RNA virus. Intriguingly, we found that C. elegans mounts a very similar transcriptional response to N. parisii and the Orsay virus, which is somewhat surprising, as these are molecularly distinct pathogens. Deciphering this novel immune/stress response to intracellular infection of epithelial cells is a major focus of the lab.
Go to full publication list
Select Publications
Troemel Lab selected publications:
Research Articles
- The purine nucleoside phosphorylase pnp-1 regulates epithelial cell resistance to infection in C. elegans.Tecle E, Chhan CB, Franklin L, Underwood RS, Hanna-Rose W, Troemel ER. PLoS Pathog. 2021 Apr 20;17(4):e1009350. doi: 10.1371/journal.ppat.1009350. eCollection 2021 Apr. PMID: 33878133
- A cullin-RING ubiquitin ligase promotes thermotolerance as part of the intracellular pathogen response in Caenorhabditis elegans. Panek J, Gang SS, Reddy KC, Luallen RJ, Fulzele A, Bennett EJ, Troemel ER. Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7950-7960. doi: 10.1073/pnas.1918417117. Epub 2020 Mar 19. PMID: 32193347
- The Caenorhabditis elegans RIG-I Homolog DRH-1 Mediates the Intracellular Pathogen Response upon Viral Infection. Sowa JN, Jiang H, Somasundaram L, Tecle E, Xu G, Wang D, Troemel ER. J Virol. 2020 Jan 6;94(2):e01173-19. doi: 10.1128/JVI.01173-19. Print 2020 Jan 6. PMID: 31619561
- Antagonistic paralogs control a switch between growth and pathogen resistance in C. elegans. Reddy KC, Dror T, Underwood RS, Osman GA, Elder CR, Desjardins CA, Cuomo CA, Barkoulas M, Troemel ER. PLoS Pathog. 2019 Jan 14;15(1):e1007528. doi: 10.1371/journal.ppat.1007528. eCollection 2019 Jan. PMID: 30640956
- An Intracellular Pathogen Response Pathway Promotes Proteostasis in C. elegans. Reddy KC, Dror T, Sowa JN, Panek J, Chen K, Lim ES, Wang D, Troemel ER. Curr Biol. 2017 Nov 20;27(22):3544-3553.e5. doi: 10.1016/j.cub.2017.10.009. Epub 2017 Nov 2. PMID: 29103937
- In vivo mapping of tissue- and subcellular-specific proteomes in Caenorhabditis elegans. Reinke AW, Mak R, Troemel ER, Bennett EJ. Sci Adv. 2017 May 10;3(5):e1602426. doi: 10.1126/sciadv.1602426. eCollection 2017 May.
- Identification of microsporidia host-exposed proteins reveals a repertoire of rapidly evolving proteins. Reinke AW*, Balla, KM, Bennett EJ, Troemel ER. Nature Communications, 2017 Jan 9;8:14023. doi: 10.1038/ncomms14023. *corresponding author.
- A Large Collection of Novel Nematode-Infecting Microsporidia and Their Diverse Interactions with Caenorhabditis elegans and Other Related Nematodes. Zhang G, Sachse M, Prevost MC, Luallen RJ, Troemel ER, Félix MA. PLoS Pathog. 2016 Dec 12;12(12):e1006093. doi: 10.1371/journal.ppat.1006093.
- Cell-to-cell spread of microsporidia causes Caenorhabditis elegans organs to form syncytia. Balla KM, Luallen RJ, Bakowski MA, Troemel ER. Nature Microbiology 1, (2016) doi:10.1038/nmicrobiol.2016.144
- Microsporidia Intracellular Development Relies on Myc Interaction Network Transcription Factors in the Host. Botts MR, Cohen LB, Probert CS, Wu F, Troemel ER. G3 (Bethesda). 2016 Jul 8. pii: g3.116.029983. doi: 10.1534/g3.116.029983. [Epub ahead of print] PMID: 27402359
- Discovery of a Natural Microsporidian Pathogen with a Broad Tissue Tropism in Caenorhabditis elegans. Luallen RJ, Reinke AW, Tong L, Botts MR, Félix MA, Troemel ER. PLoS Pathog. 2016 Jun 30;12(6):e1005724. doi: 10.1371/journal.ppat.1005724. eCollection 2016 Jun. PMID: 27362540
- The C. elegans CCAAT-Enhancer-Binding Protein Gamma Is Required for Surveillance Immunity. Reddy KC, Dunbar TL, Nargund AM, Haynes CM, Troemel ER. Cell Rep. 2016 Feb 10. pii: S2211-1247(16)30033-X. doi: 10.1016/j.celrep.2016.01.055. [Epub ahead of print] PMID:26876169
- Small GTPases promote actin coat formation on microsporidian pathogens traversing the apical membrane of C. elegans intestinal cells. Szumowski SC, Estes KA, Popovich JJ, Botts MR, Sek G, Troemel ER. Cell Microbiol. 2015 Jul 6. doi: 10.1111/cmi.12481. [Epub ahead of print] PMID:26147591
- Balla KM, Andersen EC, Kruglyak L, Troemel ER. A wild C. elegans strain has enhanced epithelial immunity to a natural microsporidian parasite. PLoS Pathogens. 2015 Feb 13;11(2):e1004583. doi: 10.1371/journal.ppat.1004583. PMID:25680197
- Szumowski SC, Botts MR, Popovich JJ, Smelkinson MG, Troemel ER. The small GTPase RAB-11 directs polarized exocytosis of the intracellular pathogen N. parisii for fecal-oral transmission from C. elegans. Proc Natl Acad Sci U S A. 2014 May 19. PMCID: PMC4050618
- Bakowski MA, Desjardins CA, Smelkinson MG, Dunbar TA, Lopez-Moyado IF, Rifkin SA, Cuomo CA, Troemel ER. Ubiquitin-Mediated Response to Microsporidia and Virus Infection in C. elegans. PLoS Pathog. 2014 Jun 19;10(6):e1004200. PMCID: PMC4063957
- Cuomo CA, Desjardins CA, Bakowski MA, Goldberg J, Ma AT, Becnel JJ, Didier ES, Fan L, Heiman DI, Levin JZ, Young S, Zeng Q, Troemel ER. Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Research 2012 Dec;22(12):2478-88. PMCID: PMC3514677
- Dunbar TL, Yan Z, Balla KM, Smelkinson MG, Troemel ER. C. elegans Detects Pathogen-Induced Translational Inhibition to Activate Immune Signaling. Cell Host Microbe. 2012 Apr 19;11(4):375-86. PMCID: PMC3334869
Reviews
- The Development of Genetic Modification Techniques in Intracellular Parasites and Potential Applications to Microsporidia. Reinke AW, Troemel ER. PLoS Pathog. 2015 Dec 31;11(12):e1005283. doi: 10.1371/journal.ppat.1005283. eCollection 2015 Dec. No abstract available. PMID:26720003
- Szumowski SC, Troemel ER. Microsporidia-host interactions. Curr Opin Microbiol. 2015 Apr 3;26:10-16. doi: 10.1016/j.mib.2015.03.006. [Epub ahead of print] Review. PMCID: PMC4577307
- Cohen LB, Troemel ER. Microbial pathogenesis and host defense in the nematode C. elegans. Curr Opin Microbiol. Curr Opin Microbiol. 2015 Feb;23:94-101. PMCID: PMC4324121
Biography
Emily Troemel received her B.S. from the University of Wisconsin-Madison and then spent a year in Japan before receiving her Ph.D. in Cell Biology at UC-San Francisco. She worked for a start-up biotech company in the Bay Area before returning to academia to do a postdoc at Massachusetts General Hospital and then joined the UCSD faculty in 2008. Since joining UCSD, she has received a Searle Scholars Award, David & Lucile Packard Foundation Award and a Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease Award.

