Single-Celled Fungi Multiply, Alien-Like, by Fusing Cells in Host
August 22, 2016
By Kim McDonald
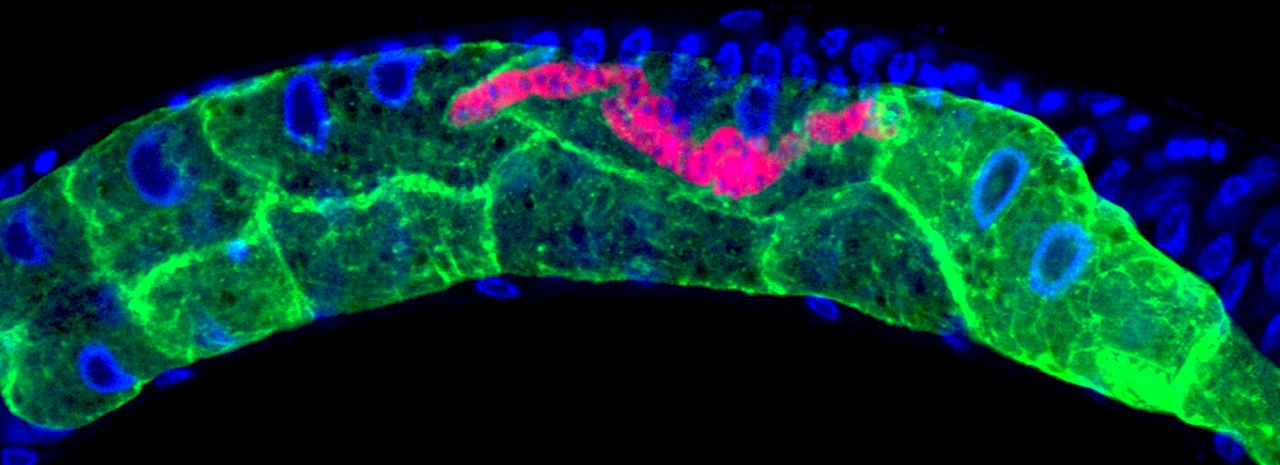
Within the transparent roundworm, microsporidia (tagged with a red fluorescent dye) multiply and spread by fusing together neighboring gut cells (shown by the green boundaries)
Keir Balla, UC San Diego
Microsporidia cause diarrhea, an illness called microsporidiosis and even death in immune-compromised individuals.
In spite of those widespread medical problems, scientists were uncertain about how these single-celled fungi reproduced in human or animal cells.
But in a study that employed transparent roundworms, biologists at the University of California San Diego succeeded in directly observing how these microorganisms replicate and spread. And what they saw surprised them.
The UC San Diego biologists report in this week’s issue of Nature Microbiology that microsporidia fuse the cells of their animal hosts together so they can multiply and quickly spread, alien-like, within their hosts’ uninfected cells.
“Viruses and bacteria have been shown to fuse host cells together to facilitate the spread of an infection,” said Emily Troemel, a professor of biology who headed the study. “But this is the first time we’ve seen this mode of infection by a eukaryotic pathogen, which is a single-celled organism with a distinct nucleus containing genetic material.”
“It’s like microsporidia have figured out that, like soldiers fighting in an urban warfare, it’s easier and safer to go from house to house by entering adjacent houses through a common wall, rather than by going through the front door of each house,” said Troemel.
Microsporidia are typically found in the intestinal tracts of animals and humans, which are made up of millions of cells. That’s one reason why studying their mode of reproduction before had been so difficult.
“We often don’t know where pathogens invade or how they spread from cell to cell,” explained Keir Balla, a graduate student working in Troemel’s laboratory and the first author of the paper. “This is partly due to the complex arrangement of cells in the body. Because the gut is made up of millions of cells, it’s virtually impossible to tell which gut cell was first invaded or the extent to which infection was able to spread. And this can prevent effective treatment.”
Although some compounds have been used to treat infections with microsporidia, none have proven effective, leading to widespread infections among humans as well as animals. A recent study conducted in the Czech Republic found that roughly one-half of the population was infected with at least one of the 14 species of microsporidia known to infect humans.
“It’s been an unappreciated medical issue because of how difficult it’s been to detect microsporidia,” said Troemel.
Balla and two other scientists in Troemel’s lab— graduate student Robert Luallen and postdoctoral fellow Malina Bakowski—used the tiny roundworm, C. elegans to figure out how microsporidia replicate from a single infection. Not only is the worm transparent, allowing the researchers to directly observe the process, but it has a very simple gut.
“The entire body of this worm is roughly 10 times shorter than a single human eyelash and its gut is made up of just 20 cells,” said Balla. “But despite the drastic differences in the number of gut cells between the worms and humans, their gut structure is quite similar. This means there are probably parallels in how microsporidian infections spread in both the human and worm cells.”
By labeling the RNA of microsporidia with a red fluorescent dye and tagging the membranes of the gut cells in green, the researchers were able to microscopically track a single infection of one microsporidia cell in one gut cell as it reproduced over two days by more than 50,000 individuals and travelled through all 20 gut cells. Thus, one parasite cell can replicate to take ultimately over the entire intestinal organ.
“While watching these infections in the living worms, we saw the microsporidia grow and spread beyond the initially invaded cell by fusing neighboring gut cells together,” said Troemel. “This process continued across all of the gut cells until the majority of the entire intestine was fused into one giant cell filled with microsporidia.”
When the researchers infected the worm’s muscle cells with microsporidia, the same process occurred. The invaders fused the cells together into one continuous structure, suggesting that the same mode of reproduction microsporidia employ in gut cells occurs in many other types of animal cells they infect—including humans.
“Our observation in this study that a single microbe can fuse many host cells into one should provide insights for other scientists designing more effective treatments for microsporidia infections,” said Balla. “Because these pathogens remain hidden within their hosts’ cells, treatments will need to be designed that deliver drugs or other agents to these intracellular regions.”
The study was supported by grants from the Institute of General Medical Sciences of the National Institutes of Health (R01GM114139) and the David and Lucile Packard Foundation.
